Contact Lenses with Controlled Drug Release
During my co-op term at the C20/20 Innovation Hub in McMaster University under the supervision of Dean of Engineering Dr. Heather Sheardown, I completed a project characterizing methacrylated vitamin E contact lenses for use as a controlled drug release system.
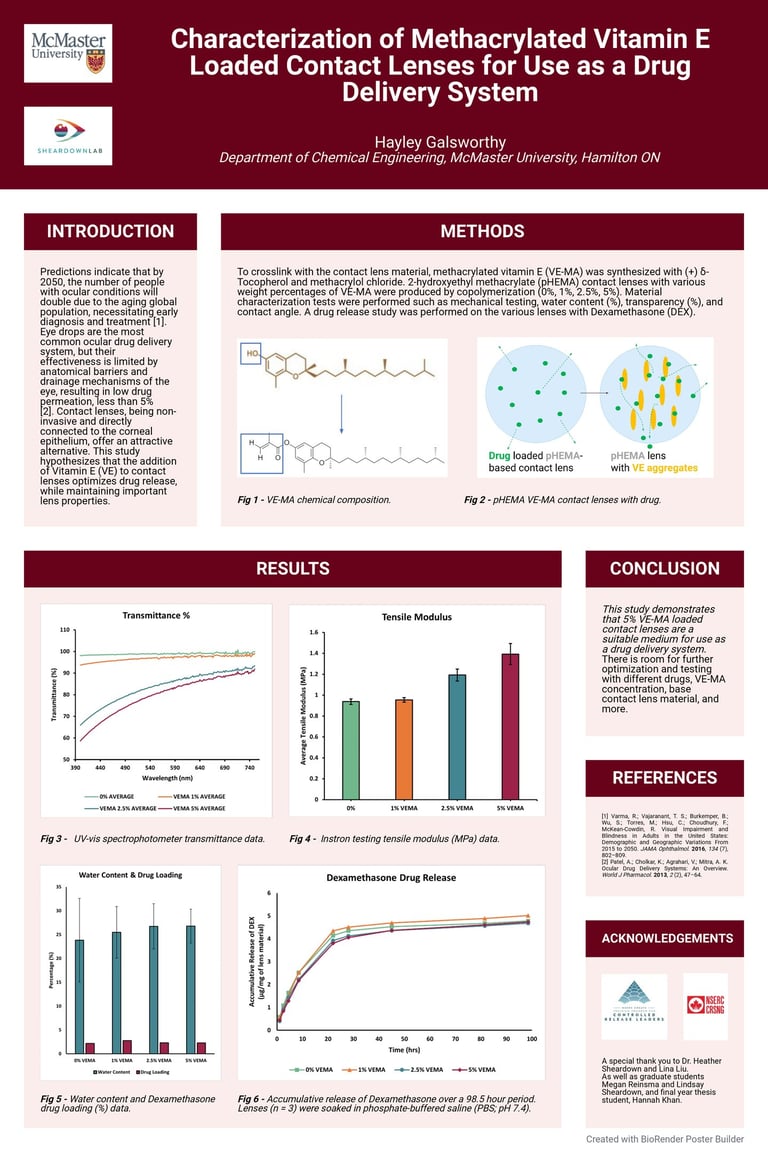
Produced a research poster summarizing findings and presented at the McMaster University’s Undergraduate Summer Research Showcase 2023.
Objective
Ocular conditions are becoming more common due to an aging global population, which has led to a greater need for effective treatments and drug delivery systems [1]. Eye drops are a common treatment, but their effectiveness is limited because they have low bioavailability and less than 5% drug permeation due to the eye's natural barriers [2].
This study explored the use of contact lenses as an alternative drug delivery method. Unlike eye drops, contact lenses have a direct connection with the corneal epithelium, which allows for more effective drug passage without leakage and can increase drug bioavailability by up to 50% [3]. By incorporating Vitamin E (VE) into contact lenses, drug release time is extended. The drug used in this study was Dexamethasone (DEX), a corticosteroid that treats eye inflammation.
Methods
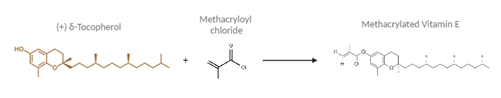
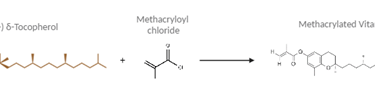
To make the contact lenses, Vitamin E was first modified into a polymerizable form called methacrylated Vitamin E (VE-MA) since the original VE monomers lack the necessary double bonds to polymerize. This was done by adding a methacrylate functional group to each monomer using methacryloyl chloride.
The research project followed these key steps to create and characterize the lenses:
Figure 1 - Synthesis of Methacrylated Vitamin E (VE-MA).
Illustration created via BioRender and ChemSpider.
Material Characterization: The fabricated lenses underwent several tests to evaluate their properties, including:
Transmittance Measurement: Used a UV-vis spectrophotometer to measure the optical transmittance of the lenses between 400 and 750 nm.
Tensile Mechanical Property Measurement: Performed on an Instron machine to determine the tensile modulus (stiffness) of the lenses.
Water Content Measurement: Measured the mass of both dry and swollen lenses to calculate their water content.
Contact Angle Measurement: A 10 µL drop of water was placed on the lens surface to record the contact angle, assessing hydrophilicity.
Drug Loading and Release: Lenses were dried, weighed, and soaked for 24 hours in a Dexamethasone solution to load the drug. The uptake percentage was calculated by re-weighing the dried, drug-loaded lenses. Drug release was measured over 98.5 hours using High-Performance Liquid Chromatography (HPLC) in a phosphate-buffered saline (PBS) solution.
Chemical Synthesis of VEMA: δ-Tocopherol, a type of Vitamin E, was dissolved in tetrahydrofuran (THF) and triethylamine (TEA) and then combined with methacryloyl chloride in a controlled, nitrogen-purged environment. The final product, VE-MA, was purified using a silica column and verified by proton nuclear magnetic resonance (H NMR).
Contact Lens Preparation: The VE-MA was added to a pre-mixed solution containing a monomer, a crosslinker, and an initiator. This mixture was then injected into a mold and cured using a 365 nm UV light for 20 minutes. The lenses were made with varying weight percentages of VE-MA: 0%, 1%, 2.5%, and 5%.
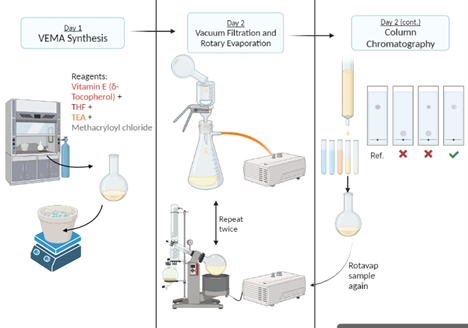
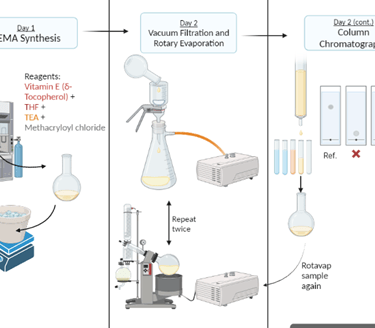
Figure 2 - Flowchart illustrating the synthesis of methacrylated Vitamin E (VE-MA). Illustration created by Hannah Khan via BioRender.
Results
Transmittance: The transmittance of both VEMA and VE lenses decreased as their concentration increased. For VEMA lenses, all concentrations (0%, 1%, 2.5%, and 5%) had acceptable transmittance values (<90%) at 690 nm. For the VE lenses, the 0%, 1%, and 2.5% concentrations were within the acceptable range.
Figure 3 - Graphs displaying the average swollen VE & VEMA % transmittance data compared to the average swollen blank % transmittance data (n = 3). All samples were placed in MilliQ water in a UV cuvette.
Drug Release: As the concentration of VEMA increased, the drug release rate from the lenses decreased. The 5% VEMA lenses showed a slightly significant difference in release rate compared to the 0% VEMA lenses, while the 1% and 2.5% VEMA lenses did not show a statistically significant difference from the 0% VEMA lenses.
Tensile Mechanical Properties: The acceptable range for a contact lens's tensile modulus is 0.1-1.9 MPa [4]. All VEMA and VE lenses were within this range. As VEMA concentration increased, the tensile modulus also increased, suggesting that VEMA strengthens the lens's lattice structure. Conversely, with VE lenses, the tensile modulus increased slightly at 1% and then decreased as the concentration went up, as VE acts as a lubricant rather than a strengthener.
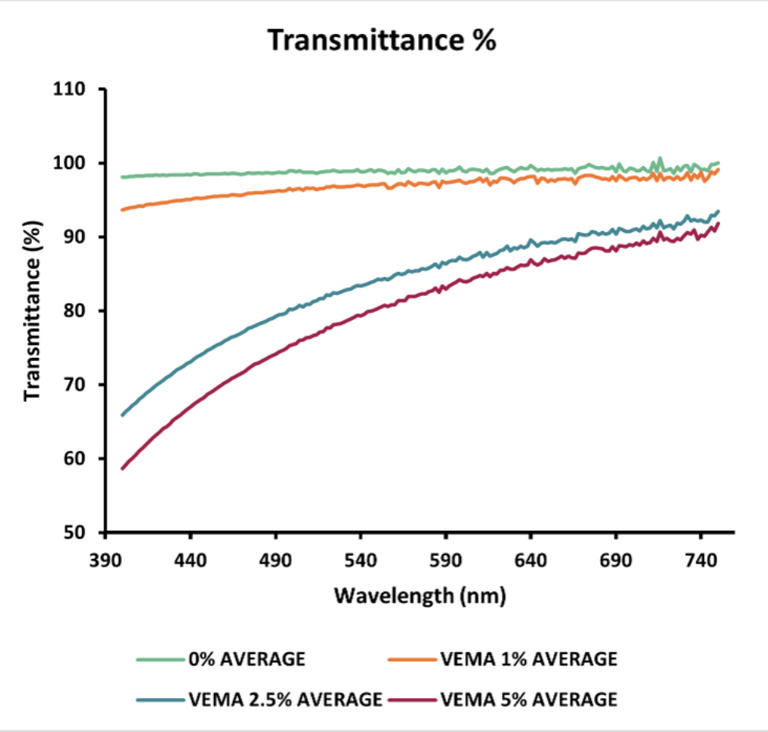
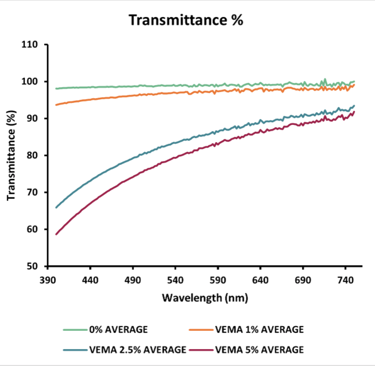
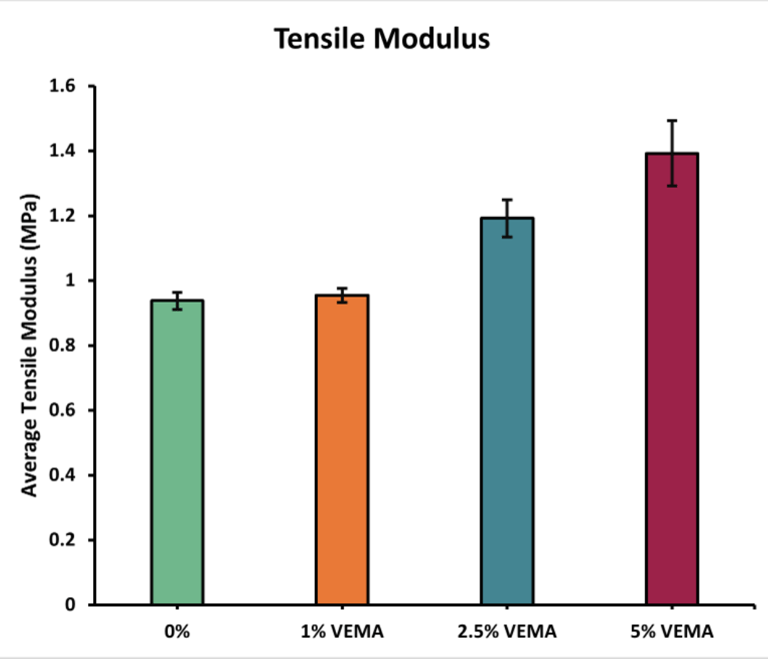
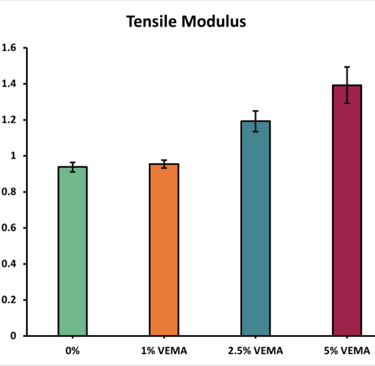
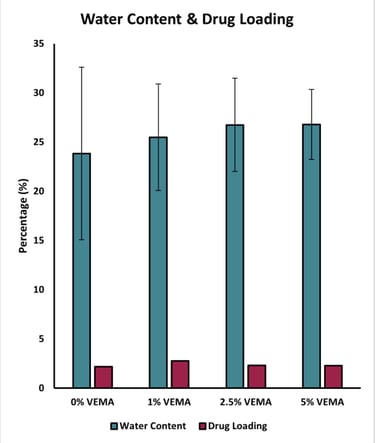
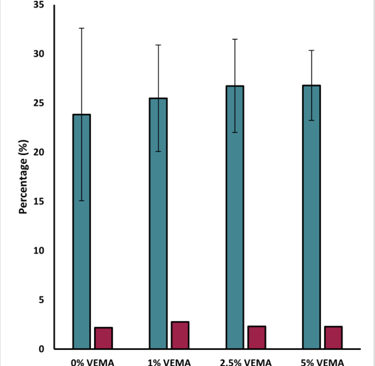
Figure 4 - Graph displaying the average tensile modulus (MPa) of the VEMA samples at various concentrations: 0%, 1%, 2.5%, and 5%. Error bars were calculated using standard error (n = 3).
Figure 5 - Graph displaying the average recorded water content and drug loading (%) of the VEMA samples at various concentrations: 0%, 1%, 2.5%, and 5%. Error bars were calculated using standard error (n = 3).
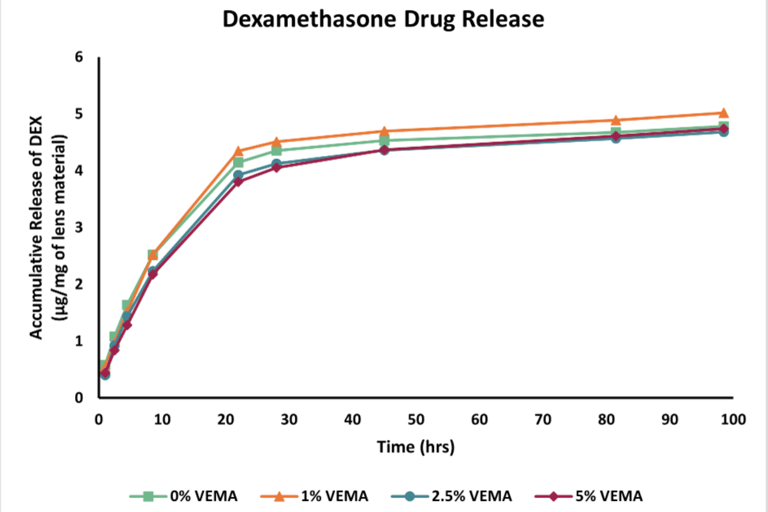
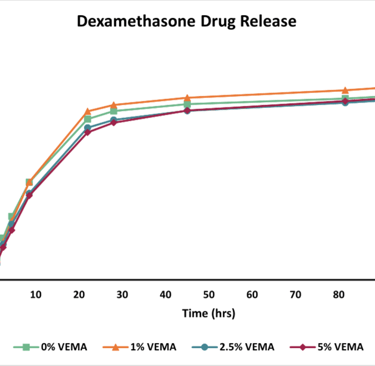
Figure 6 - Graph displaying the drug release data recorded over 98.5 hours. The drug, Dexamethasone, was quantified utilizing HPLC at 242 nm.
Conclusions
This study suggests that adding 5% VE-MA to contact lenses is the minimum concentration required to effectively slow down the drug release rate, making the lenses a suitable medium for use as a drug delivery system.
There is still significant room for further research and optimization, including:
Optimizing the concentration of VEMA
Experimenting with different base contact lens materials
Testing different types of drugs
Optimizing the drug loading method
Acknowledgements
Project builds upon the final year thesis of Hannah Khan
Research conducted under the supervision of Dr. Heather Sheardown and Lina Liu and the C20/20 Innovation Hub
Supported by the NSERC CREATE ContRoL Program
References
[1] “Visual Impairment and Blindness in Adults in the United States: Demographic and Geographic Variations From 2015 to 2050 | Ophthalmology | JAMA Ophthalmology | JAMA Network.” https://jamanetwork.com/journals/jamaophthalmology/article-abstract/2523780 (accessed Aug. 18, 2023).
[2] A. Patel, K. Cholkar, V. Agrahari, and A. K. Mitra, “Ocular drug delivery systems: An overview,” World J. Pharmacol., vol. 2, no. 2, pp. 47–64, 2013, doi: 10.5497/wjp.v2.i2.47.
[3] “Full article: A review on therapeutic contact lenses for ocular drug delivery.” https://www.tandfonline.com/doi/full/10.3109/10717544.2016.1138342 (accessed Aug. 18, 2023).
[4] T. S. Bhamra and B. J. Tighe, “Mechanical properties of contact lenses: The contribution of measurement techniques and clinical feedback to 50 years of materials development,” Contact Lens Anterior Eye, vol. 40, no. 2, pp. 70–81, Apr. 2017, doi: 10.1016/j.clae.2016.11.005.
